What Statement Describes the Best Pharmaceuticals for Children Act
Pediatric initiatives in the United States such as the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act have paved the way for an expansion in pediatric research by encouraging the pharmaceutical industry to perform necessary studies in children and having the National Institutes of Health NIH prioritize. The PD of targeted drugs in children will differ from adults.
Which statement best describes the use of report cards to compare health plans.
. The goal of BPCA is to provide better medicines for children including. Best Pharmaceuticals for Children Act. There are important age-related variations in pharmacokinetics.
In 2002 the President signed into law the Best Pharmaceuticals for Children Act which gives incentives for research on a group that has previously been considered therapeutic orphans when it. BPCA Best Pharmaceuticals for Children Act EOP2 End of Phase 2. Congress passed the Best Pharmaceuticals for Children Act BPCA of 2002 and the Pediatric Research Equity Act PREA of 2003 to encourage drug manufacturers to develop and label drugs for pediatric use.
What was the major flaw in the modernization act. 2 50 tax credit for costs of clinical testing. They provide aggregated information rather than for particular medical conditions.
Read More Slide 1. Evidence-based dosing recommendations Improved efficacy safety information Improved information in medication labels. The FDA Modernization Act contained several flaws which led to the passage of the Best Pharmaceuticals for Children Act in 2002.
He serves as a member of the NICHD-sponsored Data Monitoring Board for the Best Pharmaceuticals for Children Act and as a member of the Institute of Medicines Committee on Review of Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. However off-label drug use remains an important public health issue for infants children and adolescents because an overwhelming. T he incentives of the Best Pharmaceuticals for Children Act BPCA and the requirements of the Pediatric Research Equity Act PREA and their predecessor policies apply within a broader framework of statutes and regulations that are intended to protect public health by ensuring the safety and effectiveness of medications.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Best Pharmaceuticals for Children Act - Amends the Public Health Service Act to direct the Secretary of Health and Human Services through the National Institutes of Health NIH to develop an annual list of approved drugs for which. The incentives of the Best Pharmaceuticals for Children Act BPCA and the requirements of the Pediatric Research Equity Act PREA and their predecessor policies apply within a broader framework of statutes and regulations that are intended to protect public health by ensuring the safety and effectiveness of medications.
The Best Pharmaceuticals for Children Act of 2002 is a law mandating performance of pediatric clinical trials of off-patent and on-patent drugs. Best Pharmaceuticals For Children Act Report From The. Aliquam vulputate augue orci eget tempus dui volutpat nec.
The 2002 Best Pharmaceuticals for Children Act encourages paediatric clinical research and regulation23 The WHO launched its own programme on. Although regulatory changes including the 2002 Best Pharmaceuticals for Children Act BPCA 7 the 2003 Pediatric Research Equity Act PREA 8 the 2012 Food and Drug Administration Safety and Innovation Act FDASIA 9 and the 2017 Research to Accelerate Cures and Equity RACE for Children Act 10 have made significant strides in protecting. The PK of understudied drugs in children will differ from adults and within children according to pediatric age groups or special population.
They attract consumers to better-rated health plans. Drugs were chosen for research based on their financial benefits to. They make clear to consumers the relative importance of survival rates for various.
Disclosure Statement I have no financial relationships to disclose relating to this presentation. In the other case it required such studies in specific situations. Best Pharmaceuticals For Children Act Report From The.
Describes a 10 times fold rise in abusive head trauma during the lockdown16 Suicide ideations and attempts have increased in correlation with COVID-19 stressors17. In one case it offered marketplace incentives for the completion of pediatric drug studies. With the subsequent enactment of the Best Pharmaceuticals for Children Act BPCA in 2002 amended in 2007 and the Pediatric Research Equity Act.
1 there is a referral an approved or pending new drug application or no patent or market exclusivity protection. From the Orphan Drug Act 8 to the Best Pharmaceuticals for Children Act 9 have also employed the marketing exclusivity mechanism in order to establish incentives to engage in socially desirable activity. Gives manufactures extension of patents to evaluate drugs on the market for their safety and efficacy to children.
The following list describes license reuses offered by the National Academies Press NAP through Rightslink. Explore the pharmacodynamics PD of understudied drugs currently being administered to children. I n the late 1990s the federal government enacted policies to expand the study of drugs in children and thereby to begin to correct a serious deficit in the data on drug safety and efficacy for young patients.
Policy Framework for BPCA and PREA. Data Availability Statement. The Best Pharmaceuticals for Children Act BPCA encourages drug companies to voluntarily test drugs for pediatric safety in response to an FDA request by.
3 exclusive right to market the drug for 7 years from marketing approval date. The passage of the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act has collectively resulted in an improvement in rational prescribing for children including more than 800 labeling changes. Engineering and Medicine document the evidence-based.
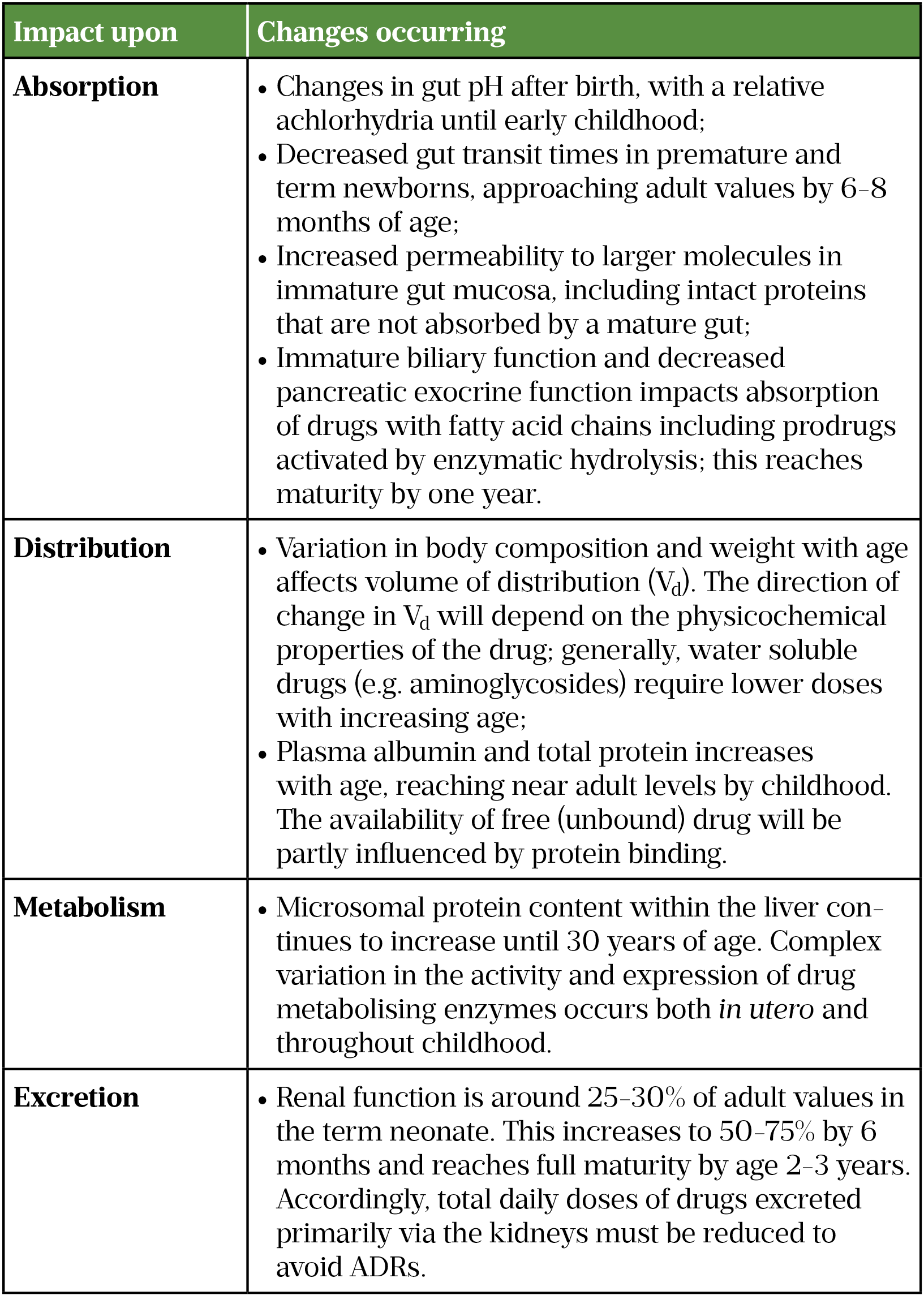
Studies done as a result of federal legislation the Best Pharmaceuticals for Children Act of 2001 and the Pediatric Research Equity Act of 2003 both made permanent in 20121 Drug dosing reference Pharmacokinetics refers to the processes of drug absorption distribution metabolism and elimination. Best Pharmaceuticals for Children Act report to accompany S. The 109 th Congress is currently considering further modifications to the existing marketing exclusivity framework in order to encourage the research and development.
The Best Pharmaceuticals for Children Act BPCA and the Pediatric Research Equity Act PREA were designed to encourage more pediatric studies of drugs used for children. 2 BPCA offers manufacturers incentives to conduct pediatric-specific research.

Recommended Strategies For The Oral Administration Of Paediatric Medicines With Food And Drinks In The Context Of Their Biopharmaceutical Properties A Review Martir 2017 Journal Of Pharmacy And Pharmacology Wiley Online Library
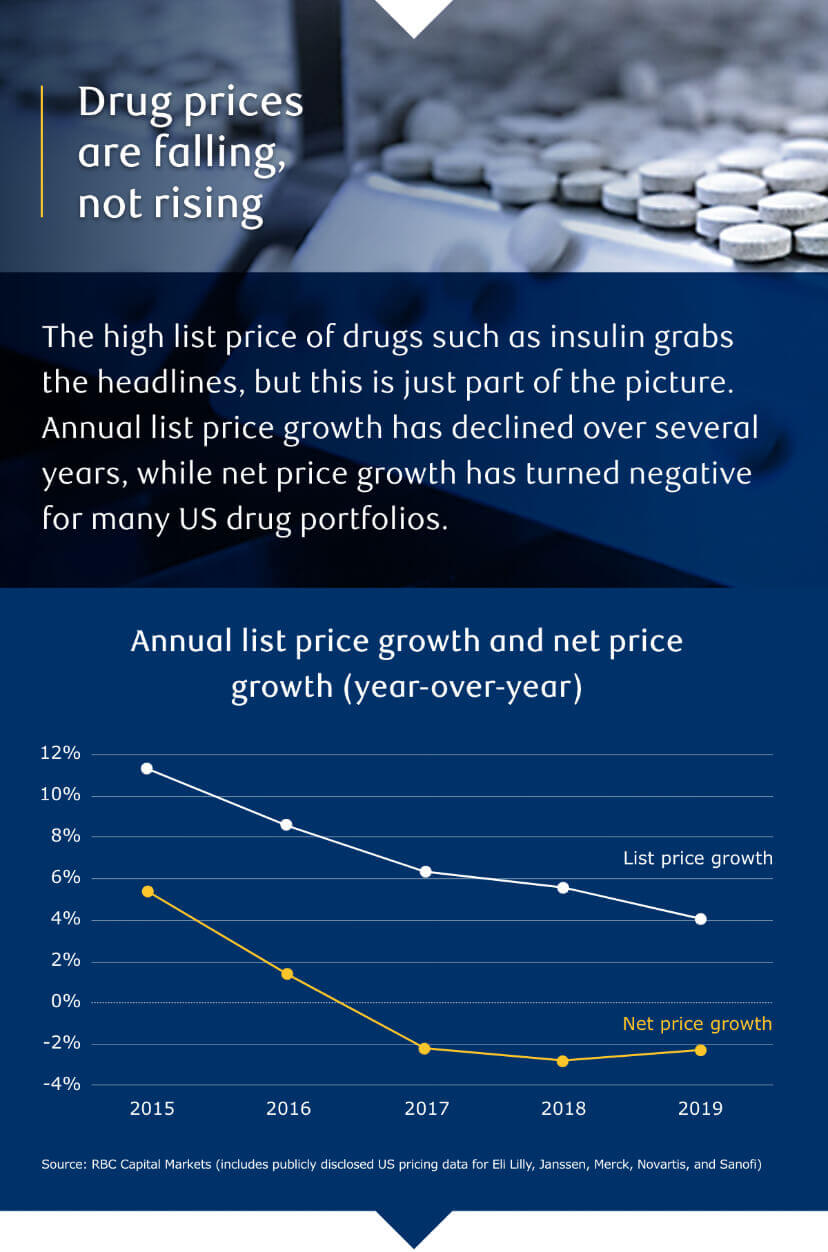
Is Pharma At An Esg Turning Point Rbc Capital Markets

Adverse Drug Reactions In Children And Young People The Pharmaceutical Journal
Comments
Post a Comment